PIPELINE > TRIAL OVERVIEW
NCT06607185
NSCLC | Pancreatic Ductal Adenocarcinoma | Colorectal Cancer | Other Solid Tumors
Pan-KRAS Inhibitor
LY4066434
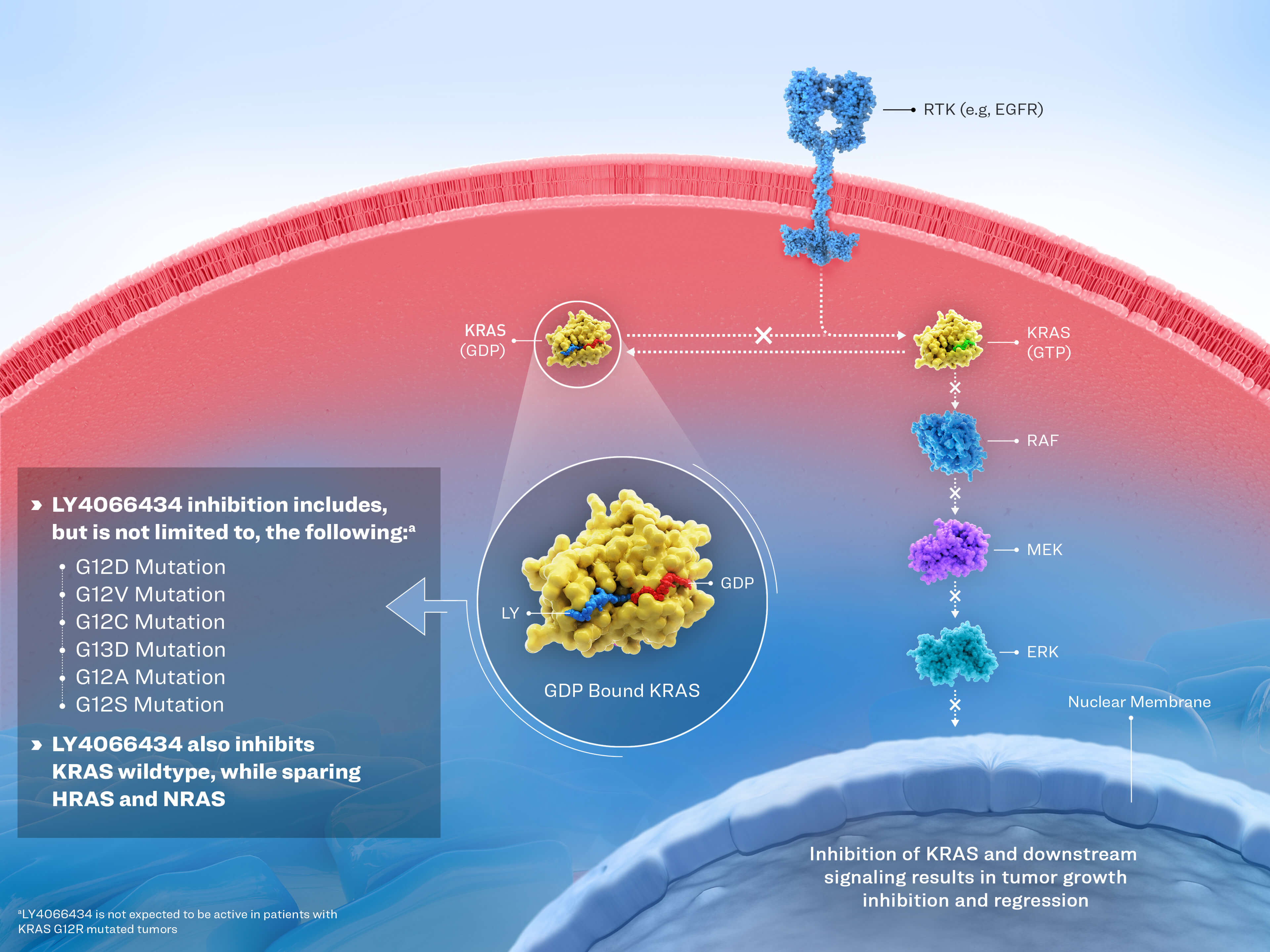
Kano Y1; Hofmann MH2; Ostrem JML and Shokat KM3; Prieto L4
Target
Molecule
Clinical Development
References
- Kano Y, et al. Nat Comm. 2019;10(1):224.
- Hofmann MH, et al. Cancer Discov. 2022;12(4):924-937.
- Ostrem JML, Shokat KM. Nat Rev Drug Discov. 2016;15(11):771-785.
- Prieto L, et al. Preclinical characterization of orally bioavailable, highly potent pan-KRAS inhibitors with selectivity over HRAS and NRAS. Presented at: AACR-NCI-EORTC Annual Meeting; October 11-15, 2023; Boston, MA. Poster B116.
- Kim D, et al. Nature. 2023;619(7968):160-166.
- Zhu G, et al. Mol Cancer. 2021;20(1):143.
- A Phase 1a/1b Study of the Pan-KRAS Inhibitor LY4066434 in Participants With KRAS Mutant Solid Tumors. ClinicalTrials.gov identifier: NCT06607185. Updated November 12, 2024. Accessed November 20, 2024. https://clinicaltrials.gov/study/NCT06607185
For information on trial enrollment, locations, and more, call
1-800-545-5979.
or visit www.clinicaltrials.gov for more information on this trial
LillyOncologyPipeline.com is
For Healthcare Professionals
The information contained in LillyOncologyPipeline.com is technical in nature and intended for Healthcare Professionals in the United States only. If you are a US Healthcare Professional, click the “Continue” button below.
Yes, I am a US Healthcare Professional and would like to continue.

