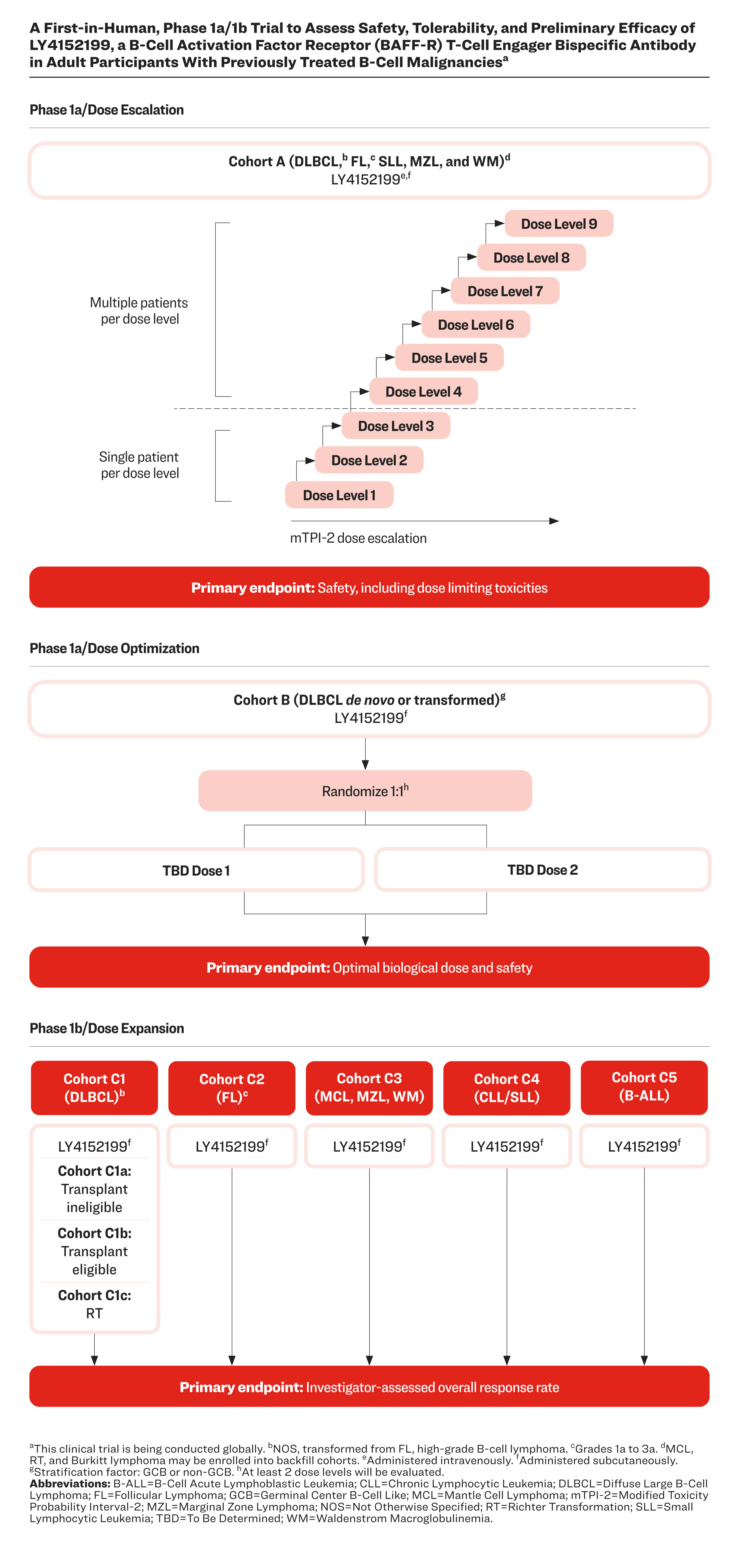
A First-in-Human, Phase 1a/1b Trial to Assess Safety, Tolerability, and Preliminary Efficacy of LY4152199, a B-Cell Activation Factor Receptor (BAFF-R) T-Cell Engager Bispecific Antibody in Adult Participants With Previously Treated B-Cell Malignanciesa